Bio-medical Applications:
Bio-medical Applications:
- tumor growth
- morphogenesis & homeostasis
- tissue bending
- drug & biomarkers penetration
- circulating tumor cells biomechanics
Modeling Techniques:
IBCell model:
IBCell Model: an Immersed Boundary Model of the Cell
A biomechanical model of fully deformable cells comprising of an elastic membrane that provides cell shape with viscous incompressible cytoplasm providing cell mass. Due to local interactions with other cells and with the microenvironment, that take place via cell membrane receptors, individual cells can self-organize in multicellular structures and act together as one tissue.
- K.A. Rejniak, "An immersed boundary framework for modelling the growth of individual cells: an application to the early tumour development", Journal of Theoretical Biology, 2007, 247:186-204, Pubmed ID#17416390; link
- K.A. Rejniak, S.E. Wang, N.S. Bryce, H. Chang, B. Parvin, J. Jourquin, L. Estrada, J.W. Gray, C.L. Arteaga, A.M. Weaver, V. Quaranta, A.R.A. Anderson, "Linking changes in epithelial morphogenesis to cancer mutations using computational modeling" PLoS Computational Biology, 2010, 6(8):e1000900, Pubmed ID#20865159, PMCID:PMC2928778, open access
- K.A. Rejniak, "Homeostatic imbalance in epithelial ducts and its role in carcinogenesis", Scientifica, ID:132978, 2012, Pubmed ID#24278670, PMCID:PMC3820568, open access
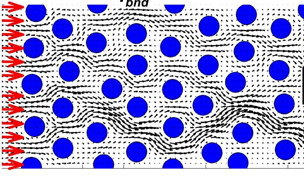
microPD model:
microPD Model: a micro-scale model of drug pharmacodynamics
A small (a few hundred of microns in length) two-dimensional patch of the tumor tissue is considered with explicitly defined tissue morphology composed of individual tumor cells embedded in the ECM and surrounded by interstitial space filled with fluid. The drug (either individual drug molecules or drug concentrations) are transported (both by diffusion and advection) through the interstitial space and can by taken up by the cells to exert their therapeutic effect.
- K.A. Rejniak, V. Estrella., T. Chen., A.S. Cohen., M.C. Lloyd, D.L. Morse,"The role of tumor tissue architecture on treatment penetration and efficacy: an integrative study", Frontiers in Oncology, Special Topic Title: Computational Models in Oncology: from Tumor Initiation to Progression to Treatment, Host Speciality: Frontiers in Molecular and Cellular Oncology, Frontiers in Oncology Journal, 3:111, 2013, Pubmed ID#23717812, PMCID:PMC3650652, open access
- M.J. Kim, R.J. Gillies, K.A. Rejniak, "Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues", Frontiers in Oncology, Special Topic Title: Ways to improve tumor uptake and penetration of drugs into solid tumors, Host Specialty: Frontiers in Pharmacology of Anti-Cancer Drugs, Frontiers in Oncology Journal, 3:278, 2013, Pubmed ID#24303366, PMCID:PMC3831268, open access
![]()
Model of circulating tumor cells (CTC):
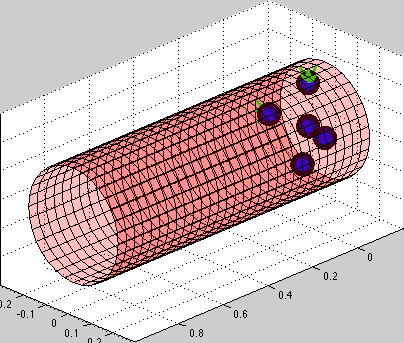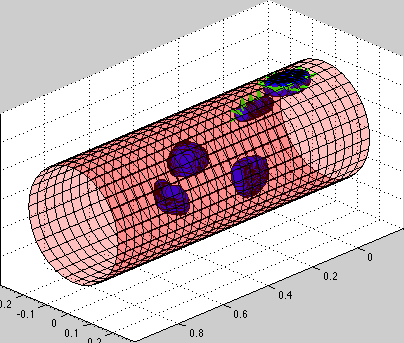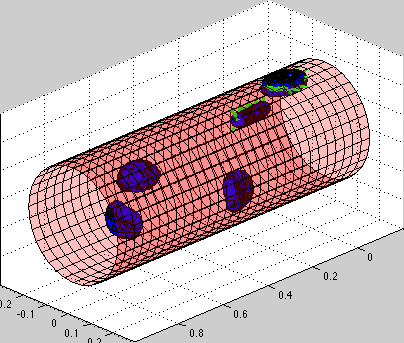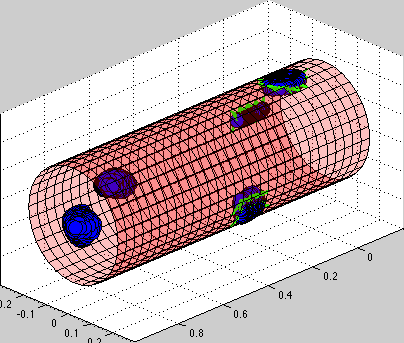
circulating tumor cells biomechanics
- K.A. Rejniak, "Investigating dynamical deformations of tumor cells in circulation: predictions from a theoretical model", Frontiers in Oncology, Special Topic Title: Therapeutic targeting of circulating tumor cells, Host Specialty: Frontiers in Cancer Molecular Targets and Therapeutics, Frontiers in Oncology Journal, 2:111, 2012, Pubmed ID#23024961, PMCID:PMC3444760, open access
![]()
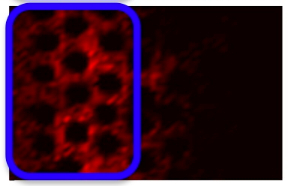
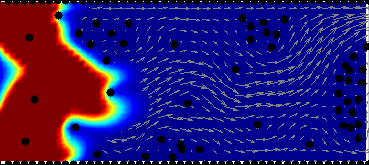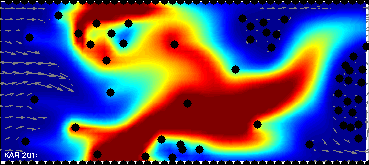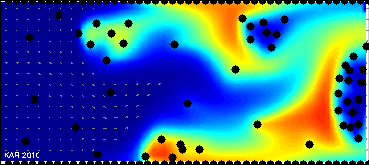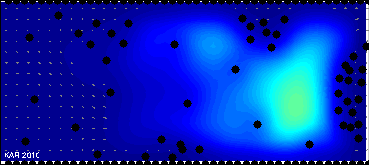
Model of drug and biomarkers penetration:
drug & biomarkers penetration through the tissue
- K.A. Rejniak, V. Estrella., T. Chen., A.S. Cohen., M.C. Lloyd, D.L. Morse,"The role of tumor tissue architecture on treatment penetration and efficacy: an integrative study", Frontiers in Oncology, Special Topic Title: Computational Models in Oncology: from Tumor Initiation to Progression to Treatment, Host Speciality: Frontiers in Molecular and Cellular Oncology, Frontiers in Oncology Journal, 3:111, 2013, Pubmed ID#23717812, PMCID:PMC3650652, open access
- M.J. Kim, R.J. Gillies, K.A. Rejniak, "Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues", Frontiers in Oncology, Special Topic Title: Ways to improve tumor uptake and penetration of drugs into solid tumors, Host Specialty: Frontiers in Pharmacology of Anti-Cancer Drugs, Frontiers in Oncology Journal, 3:278, 2013, Pubmed ID#24303366, PMCID:PMC3831268, open access
![]()
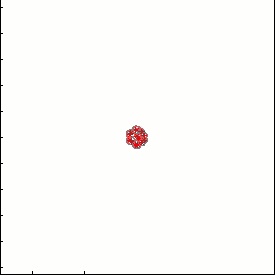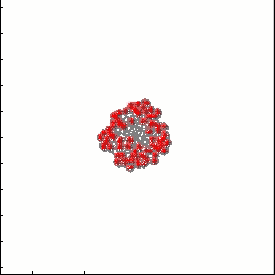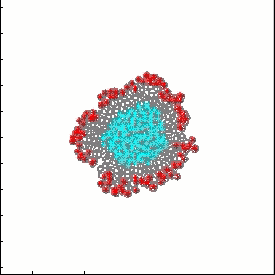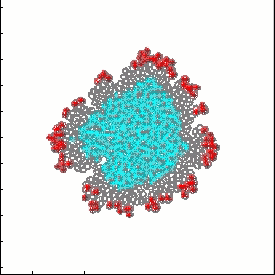
Model of tumor growth and invasion:
tumor growth
- K.A. Rejniak, "An immersed boundary framework for modelling the growth of individual cells: an application to the early tumour development", Journal of Theoretical Biology, 2007, 247:186-204, Pubmed ID#17416390; link
- K.A. Rejniak, "A single cell approach in modeling the dynamics of tumor microregions", Mathematical Biosciences and Engineering, 2005, 2(3):643-655, Pubmed ID#20369945; link
- K.A. Rejniak, R.H. Dillon, "A single cell based model of the ductal tumour microarchitecture", Computational and Mathematical Methods in Medicine, 2007 8(1):51-69, open access
- A.R.A. Anderson, K.A. Rejniak, P. Gerlee, V. Quaranta, "Microenvironment driven invasion: a multiscale multimodel investigation", Journal of Mathematical Biology, 2009, 58:579-624, Pubmed ID#18839176; link
- V. Quaranta, K.A. Rejniak, P. Gerlee, A.R.A. Anderson, "Invasion emerges from cancer cell adaptation to competitive microenvironments: quantitative predictions from multiscale mathematical models", Seminars in Cancer Biology, 2008, 18:338-348, Pubmed ID#18524624, PMCID:PMC3789515, Free PMC Article
![]()
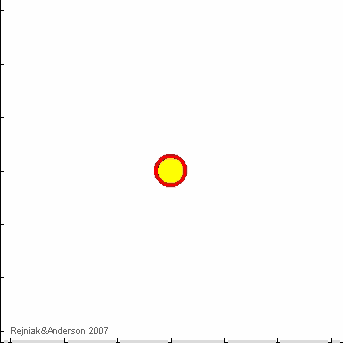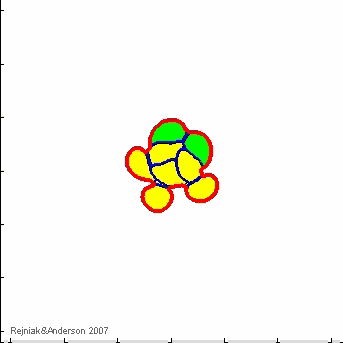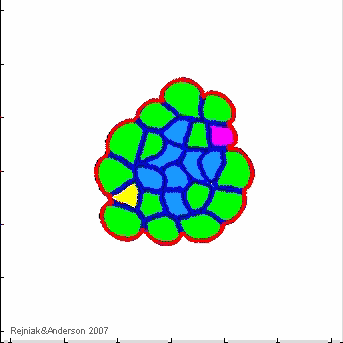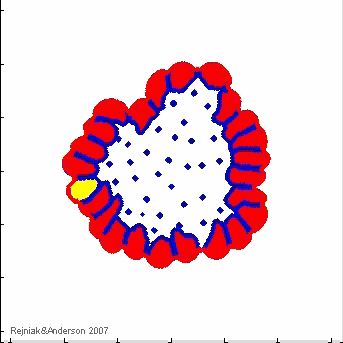
Model of morphogenesis & homeostasis:
morphogenesis & homeostasis
- K.A. Rejniak, "An immersed boundary framework for modelling the growth of individual cells: an application to the early tumour development", Journal of Theoretical Biology, 2007, 247:186-204, Pubmed ID#17416390; link
- K.A. Rejniak, S.E. Wang, N.S. Bryce, H. Chang, B. Parvin, J. Jourquin, L. Estrada, J.W. Gray, C.L. Arteaga, A.M. Weaver, V. Quaranta, A.R.A. Anderson, "Linking changes in epithelial morphogenesis to cancer mutations using computational modeling" PLoS Computational Biology, 2010, 6(8):e1000900, Pubmed ID#20865159, PMCID:PMC2928778, open access
- K.A. Rejniak, "Homeostatic imbalance in epithelial ducts and its role in carcinogenesis", Scientifica, ID:132978, 2012, Pubmed ID#24278670, PMCID:PMC3820568, open access
![]()
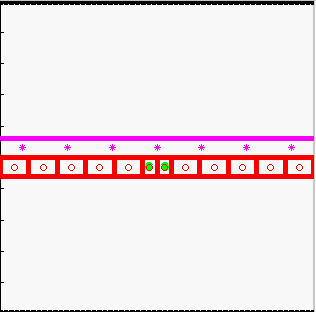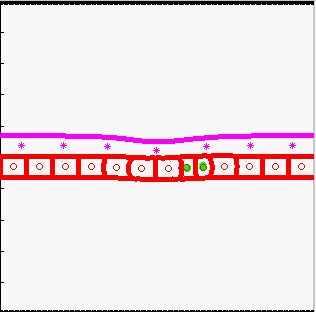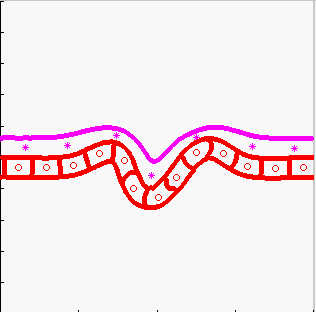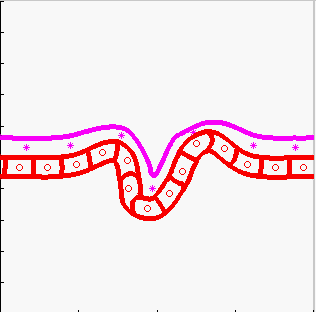
Model of tissue bending:
tissue bending: trophoblast
- K.A. Rejniak, H.J. Kliman, L.J. Fauci, "A computational model of the mechanics of growth of the villous trophoblast bilayer", Bulletin of Mathematical Biology, 2004, 66(2):199-232, Pubmed ID#14871565; link
![]()
Immersed boundary method:
Immersed Boundary framework for IBCell
This classic fluid-structure interaction method couples the mechanics of immersed elastic bodies (here: cells) with the dynamics of a viscous incompressible fluid (here: the cytoplasm and the extracellular matrix). The strength of the immersed boundary method is that it can handle the complicated and time dependent geometries of individual elastic cells which can be arranged into tissues of different topologies, and that it does so while using a fixed regular lattice for all fluid computations.
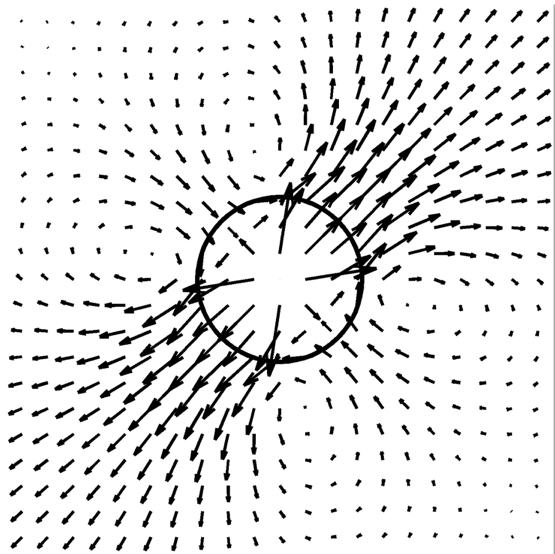
Regularized Stokeslet method:
Regularized Stokeslets framework for microPD
This classic fluid-structure inteaction method allows for computing the fluid flow at low Reynolds numbers that is influenced by both mobile and stationary, deformable or solid objects, as well as for solving the inverse problem, that is, computing the forces that will lead to a desire object velocities.